research
STRUCTURE ELUCIDATION OF PROTEINS
AND COMPLEXES BY MASS SPECTROMETRY
Our group is interested in developing and applying new tools based on electrospray ionization mass spectrometry (ESI-MS) and ion mobility to detect and to characterize the structures of proteins and protein complexes. “Native” MS, or the direct measurement of biomolecules from near-physiological solutions by MS, is a major focus of our research efforts.
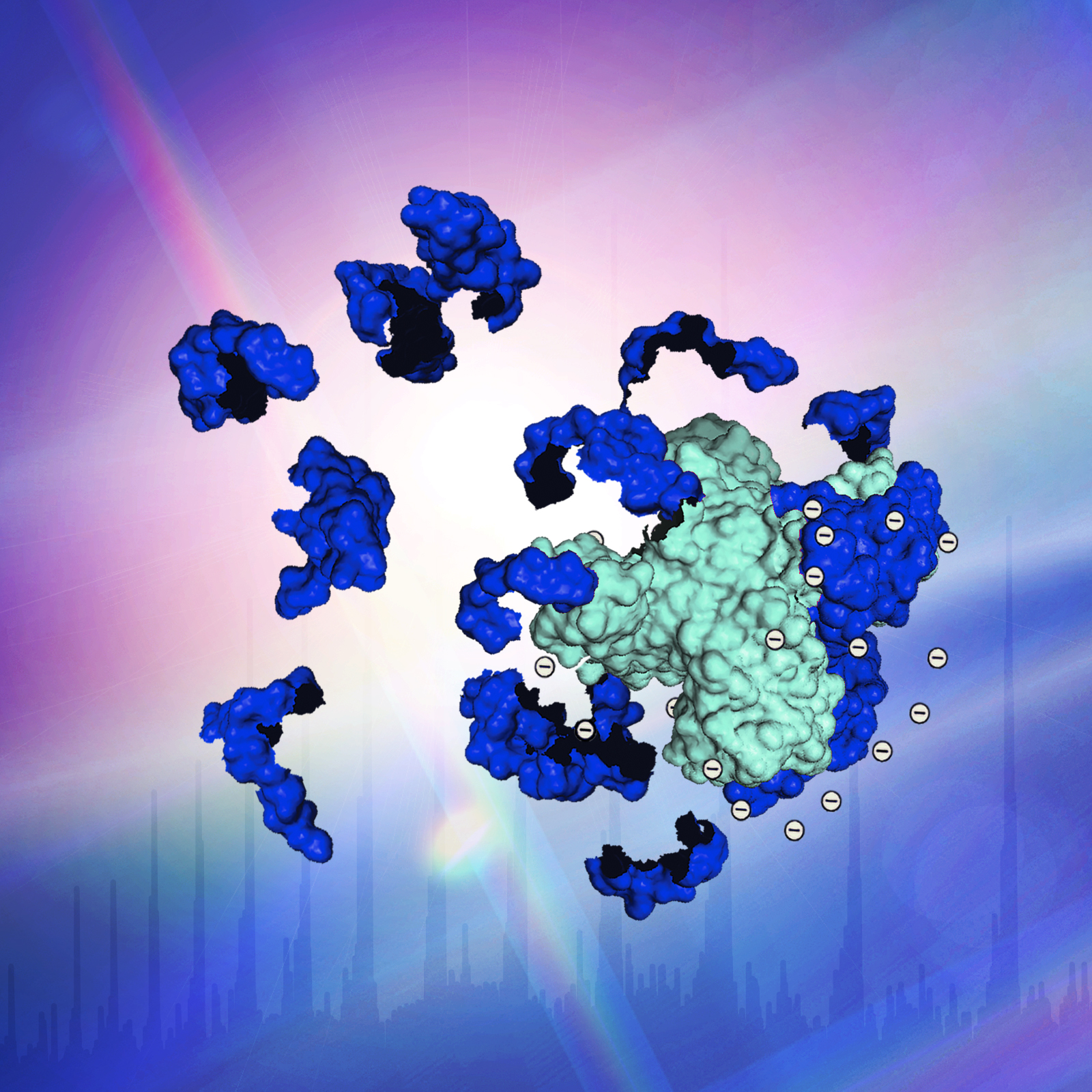
We are developing MS methods to provide molecular site-specific information. Using “top-down” MS, the direct MS-sequencing of large molecules (without digestion into smaller fragments), we developed an MS-only based method to locate protein-ligand binding sites, and we have extended this work to address the sites of binding of a novel protein aggregation inhibitor that may have application in neurodegenerative diseases.
Using high resolution MS with various forms of dissociation methods (electron capture dissociation, photodissociation), we have extended top-down MS to address larger protein-ligand and protein-protein complexes. Top-down MS of protein complexes yields sequence information primarily from the outer surface of the complex, suggesting a unique utility for structural biology. Direct information on the presence of disulfide bonds, modifications, and “proteoforms” can be obtained by top-down MS. Our future research will continue to advance mass spectrometry as a tool for structural biology, especially for membrane proteins, and the areas of neurodegenerative diseases and aging.
Representative publications
1. Xie Y, Zhang J, Yin S, and Loo JA. “Top-Down ESI-ECD-FT-ICR Mass Spectrometry Localizes Noncovalent Protein-Ligand Binding Sites.” J Am Chem Soc 2006; 128: 14432-14433.
2. Kaddis CS, Lomeli SH, Yin S, Berhane B, Apostol MI, Kickhoefer VA, Rome LH, and Loo JA. “Sizing Large Proteins and Protein Complexes by Electrospray Ionization Mass Spectrometry and Ion Mobility.” J Am Soc Mass Spectrom 2007; 18: 1206-1216.
3. Yin S and Loo JA. “Top-Down Mass Spectrometry of Supercharged Native Protein-Ligand Complexes.” Int J Mass Spectrom 2011; 300: 118-122.
4. Sinha S, Attar A, Maiti P, Du Z, Tan M, Pang ES, Lopes DHJ, Tseng C-H, Jones MR, Talbiersky P, Tennstädt A, Lichti-Kaiser K, Liu T, Yang F, Bakshi R, Kuo P-Y, Lomakin A, Gale GD, Benedek GB, Staudinger JL, Ehrmann M, Loo JA, Xie C-W, Klärner F-G, Schrader T, Wang C, Frautschy SA, and Bitan G. “Lysine-specific molecular tweezers are broad-spectrum inhibitors of assembly and toxicity of amyloid proteins.” J Am Chem Soc 2011; 133: 16958–16969.
5. Spirig T, Malmirchegini GR, Zhang J, Robson S, Sjodt M, Liu M, Kumar KK, Dickson CF, Gell DA, Lei B, Loo JA, and Clubb RT. “Staphylococcus aureus uses a novel multidomain receptor to break apart human hemoglobin and steal its heme.” J Biol Chem 2013; 288: 1065-1078.
6. Acharya S, Safaie BM, Wongkongkathep P, Ivanova MI, Attar A, Klärner F-G, Schrader T, Loo JA, Bitan G, and Lapidus LJ. “Molecular Basis for Preventing α-Synuclein Aggregation by a Molecular Tweezer.” J Biol Chem 2014; 289: 10727-10737.
7. Li H, Wolff JJ, Van Orden SL, and Loo JA. “Native Top-Down Electrospray Ionization-Mass Spectrometry of 158 kDa Protein Complex by High-Resolution Fourier Transform Ion Cyclotron Resonance Mass Spectrometry.” Anal Chem 2014; 86: 317−320.
8. Li H, Wongkongkathep P, Van Orden SL, Ogorzalek Loo RR, and Loo JA. “Revealing Ligand Binding Sites and Quantifying Subunit Variants of Non-Covalent Protein Complexes in a Single Native Top-Down FTICR MS Experiment.” J Am Soc Mass Spectrom 2014; 25: 2060-2068.